Content
Van der Waals Forces
Electron Matter
Insulator and Conductor
Insulator
Conductor
A Model of a Metal
Metal in Electric Field
Drude Model of Electron Motion in a Metal
Difference between Conductor and Insulator
Source and Reference
Van der Waals Forces
Induced dipoles cause weak attraction. Electron clouds of atoms and molecules fluctuate continually. They make temporary dipoles. They see each other, fluctuate together, and attract each other more.Electron Matter
Electrons inside materials have their own phases of matter and phase transitions. e.g. Metal, insulator, magnet, superconductor.Insulator and Conductor
Insulator: charges are bound to the atoms or molecules. Conductor: charges can flow throughout the material.Insulator
 Under a electric field, each electron shifts slightly (<1Å), but the net effect can be large.
The polarization inside a material due to the total electric field is
Under a electric field, each electron shifts slightly (<1Å), but the net effect can be large.
The polarization inside a material due to the total electric field is
𝑝
=𝛼(𝐸
𝑎𝑝𝑝𝑙𝑖𝑒𝑑+𝐸
𝑑𝑖𝑝𝑜𝑙𝑒𝑠)𝐸
𝑎𝑝𝑝𝑙𝑖𝑒𝑑≫𝐸
𝑑𝑖𝑝𝑜𝑙𝑒𝑠⇒𝑝
=𝛼𝐸
𝑎𝑝𝑝𝑙𝑖𝑒𝑑𝐸𝑎𝑝𝑝𝑙𝑖𝑒𝑑 or for low density of dipoles.
Conductor
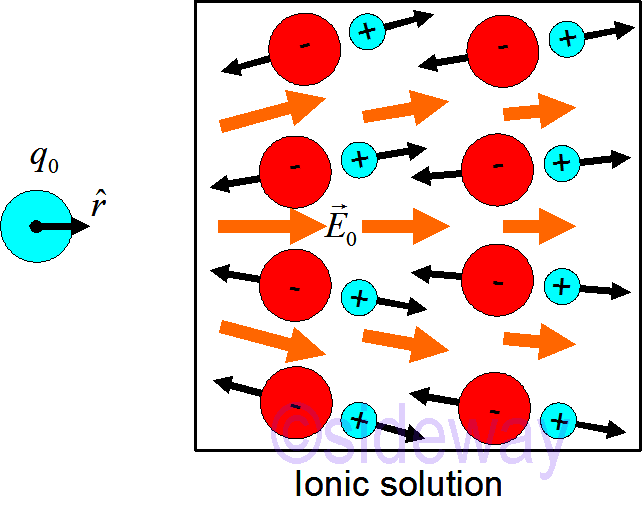 Examples of conductors: metals, ionic solutions. Unlike insulator, charges flow like liquid in a conductor. By applying an external point charge, the original ionic equilibrium state of the conductor is broken by the electric field due to the point charge. Mobile charges inside the conductor begin to move by the exerted electric force due to the applied electric field. Polarization occurs when the moving of mobile charges begin. Mobile charges will pile up in one location and the concentration of charge will create an electric field in the region. At any location inside the conductor, the mobile charge particle always experence both the applied electric field
Examples of conductors: metals, ionic solutions. Unlike insulator, charges flow like liquid in a conductor. By applying an external point charge, the original ionic equilibrium state of the conductor is broken by the electric field due to the point charge. Mobile charges inside the conductor begin to move by the exerted electric force due to the applied electric field. Polarization occurs when the moving of mobile charges begin. Mobile charges will pile up in one location and the concentration of charge will create an electric field in the region. At any location inside the conductor, the mobile charge particle always experence both the applied electric field 𝐸𝑎𝑝𝑝𝑙𝑖𝑒𝑑 and the polarization electric field
𝐸𝑝𝑜𝑙𝑎𝑟. The resultant electric field
𝐸𝑛𝑒𝑡 will exert an electric force on the mobile charge and drive the mobile charge in the direction of the force. Mobile charges will move until the conductor is in equilibrium, i.e.
𝐸𝑛𝑒𝑡=0.
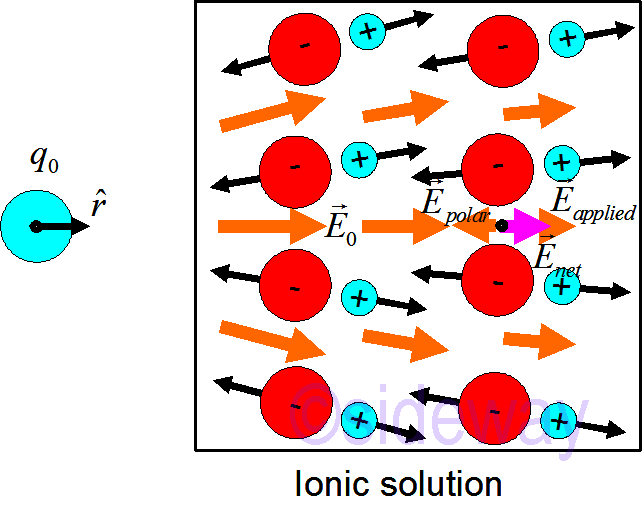 This can be proved by contradiction. Assume
This can be proved by contradiction. Assume 𝐸𝑛𝑒𝑡≠0 in static equilibrium. If
𝐸𝑛𝑒𝑡≠0, charges will move. This is not equilibrium. Tje assumption
𝐸𝑛𝑒𝑡≠0 is self-contradictory. Therefore
𝐸𝑛𝑒𝑡=0 inside a conductor in static equilibrium.
A Model of a Metal
 The atoms in a metal are arranged in a regular 3D lattice. The inner electrons of each metal atom are bound to the nucleus. Some of the outer electrons act as chemical bonding electrons and some of the outer electrons are mobile electron. These mobile electrons are free to move throughout the conductor and form the sea of mobile electrons of conductor. A 2D section of a unpolarizeed conductor is as
following.
The atoms in a metal are arranged in a regular 3D lattice. The inner electrons of each metal atom are bound to the nucleus. Some of the outer electrons act as chemical bonding electrons and some of the outer electrons are mobile electron. These mobile electrons are free to move throughout the conductor and form the sea of mobile electrons of conductor. A 2D section of a unpolarizeed conductor is as
following.

Metal in Electric Field
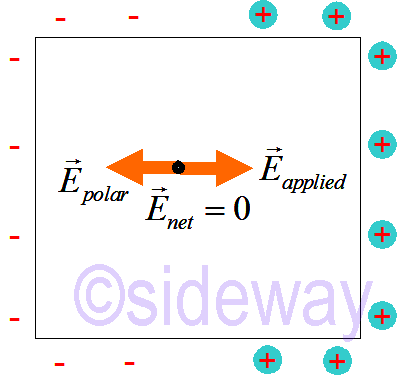
When applying an electric field to a metal conductor, moble electron sea will shift opposite to the direction of the applied electric field.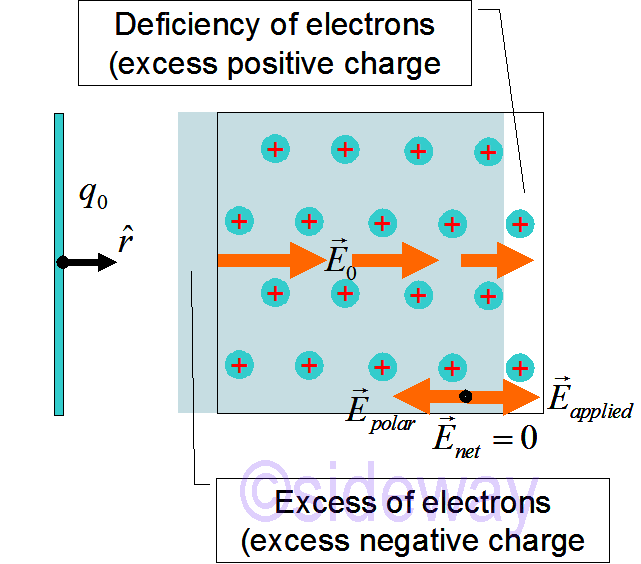 Excess charges in any conductor are always found on the surface, while the net electric field inside the conductor is still equal to zero. The net charge of the metal conductor is still equal to zero. The metal conductor can be simplified as
Excess charges in any conductor are always found on the surface, while the net electric field inside the conductor is still equal to zero. The net charge of the metal conductor is still equal to zero. The metal conductor can be simplified as
Drude Model of Electron Motion in a Metal
Mobile electrons always collide with defects or wigglin atoms. Negligible net interaction between mobile electrons and forget previous velocity after collision and start a new motion at zero velocity. By the momentum principle∆𝑝∆𝑡
=𝐹𝑛𝑒𝑡=𝑞𝐸𝑛𝑒𝑡=(−𝑒)𝐸𝑛𝑒𝑡
∆𝑝=𝑝−0=𝑒𝐸𝑛𝑒𝑡∆𝑡
𝑣=𝑝𝑚𝑒=𝑒𝐸𝑛𝑒𝑡∆𝑡𝑚𝑒
The average velocity 𝑣=𝑒∆𝑡𝑚𝑒
𝐸𝑛𝑒𝑡≡𝜇𝐸𝑛𝑒𝑡 where 𝜇 is mobility
Difference between Conductor and Insulator
Source and Reference
https://www.youtube.com/watch?v=GJiUoP5ldgA&list=PLZ6kagz8q0bvxaUKCe2RRvU_h7wtNNxxi&index=4
©sideway
ID: 191101502 Last Updated: 11/15/2019 Revision: 0
Latest Updated Links
- Travel Singapore Sight Space(last updated On 12/30/2025)
- Travel Singapore Sight Curiosity Cove(last updated On 12/30/2025)
- Travel Singapore Sight Night Safari(last updated On 12/30/2025)
- Travel Singapore Sight River Wonders(last updated On 12/30/2025)
- Travel Singapore Sight Rainforest Wild ASIA(last updated On 12/30/2025)
- Travel Singapore Sight Singapore Zoo(last updated On 12/30/2025)
- Travel Singapore Sight Mandai(last updated On 12/30/2025)
- Travel Singapore Sight Bird Paradise(last updated On 12/30/2025)
- Travel Singapore Sight AltitudeX(last updated On 12/30/2025)
- Travel Singapore Sight(last updated On 12/6/2025)
- Travel Singapore Rail Network(last updated On 12/5/2025)

 Nu Html Checker
Nu Html Checker  53
53  na
na  na
na
Home 5
Business
Management
HBR 3
Information
Recreation
Hobbies 9
Culture
Chinese 1097
English 339
Travel 31
Reference 79
Hardware 54
Computer
Hardware 259
Software
Application 213
Digitization 37
Latex 52
Manim 205
KB 1
Numeric 19
Programming
Web 289
Unicode 504
HTML 66
CSS 65
SVG 46
ASP.NET 270
OS 431
DeskTop 7
Python 72
Knowledge
Mathematics
Formulas 8
Set 1
Logic 1
Algebra 84
Number Theory 206
Trigonometry 31
Geometry 34
Calculus 67
Engineering
Tables 8
Mechanical
Rigid Bodies
Statics 92
Dynamics 37
Fluid 5
Control
Acoustics 19
Natural Sciences
Matter 1
Electric 27
Biology 1
